The introduction of this new French regulation for medical environments, means signifcant changes in the design of mechanical, thermostatic and electronic mixers.
DELABIE is among the few manufacturers to have kept up with these developments and design products with the necessary technology to meet the requirements.

At DELABIE we pride ourselves in being experts in making mixers and taps for healthcare establishments across the world, taking into account the different regulations and norms in each country as we embark on the design of each new control.
In France, the introduction of new regulations for medical settings means big changes in the design of mechanical, thermostatic and electronic mixers. What are the changes that have been made? Why? And what can we learn?
The importance of sanitary regulations in healthcare establishments is well-known. Bacterial contamination of water systems carries an extremely high risk to the health of patients with weakened immune systems, principally linked to the proliferation of Legionella and Pseudomonas aeruginosa.
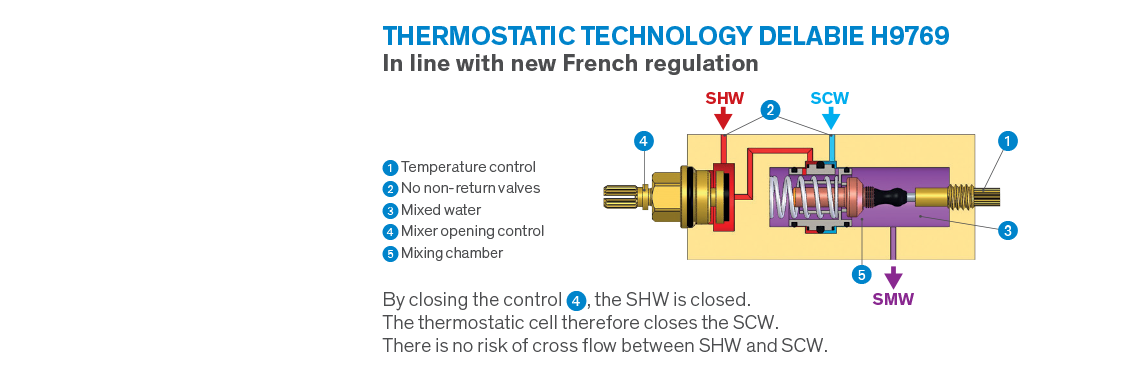
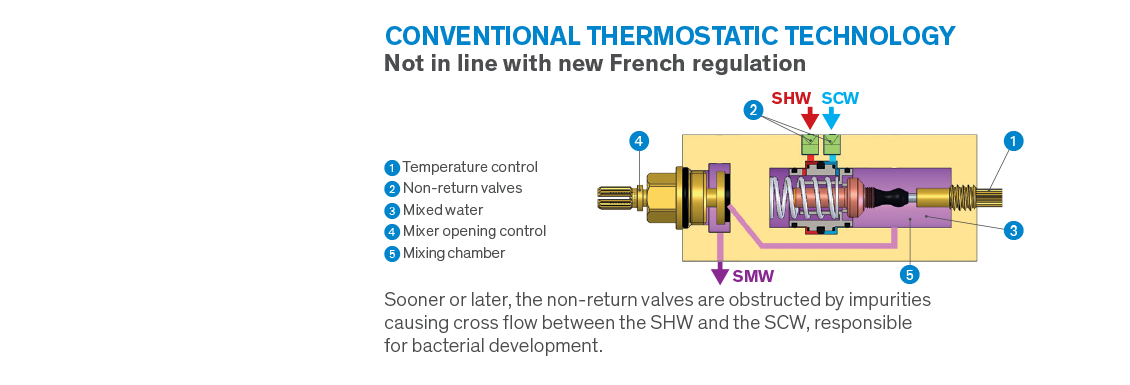
In France a new regulation, known as NF MM, was recently introduced, establishing a whole new set of specific requirements in the healthcare sector. Some of these requirements are not surprising and are already commonplace in the UK, however, the decision to ban controls with a mixing chamber* under pressure upstream of the closing mechanism is a radical change, and significantly reduces the number of compliant mixers.
*where the mixing of hot and cold water takes place in order to obtain mixed water
The end of non-return valves
Cross flow between hot and cold water inevitably creates conditions favourable to bacterial development in water controls. In order to avoid this risk, all conventional thermostatic mixers have non-return valves. However, non-return valves are not reliable because sooner or later impurities prevent them from being watertight meaning they cannot function correctly. In the event of failure, they are responsible for SHW/SCW cross flow causing incurable conditions favourable to bacterial development inside mixers. This is why French regulators have decided to outlaw these types of controls.
With the obligation to have mixers’ closing mechanisms located upstream of mixing chambers, eliminating the need for non-return valves, the French regulation NF MM is revolutionising the market. This regulation rules out the majority of electronic and thermostatic mixers produced for medical settings.

EPDM flexibles and aerators definitively banned
In terms of supplies to mixers the objective of the norm is to, barring being able to prevent, delay the formation of biofilm (a group of microorganisms that form a thin viscous layer).
Single hole deck-mounted controls must therefore be fitted with either copper tails, or pex or silicone flexible hoses, for the hot and cold water supplies.
Generally used in domestic installations, EPDM flexibles are not suitable for healthcare establishments. Both preventative and curative treatments cause this material to rapidly deteriorate. After repeated thermal or chemical shocks, the EPDM hose releases debris which also favours bacterial development.
Also banned, aerators, which mix water and air generated at the tap or mixer outlet, increase the risk of creating aerosols. This is before taking into account the impurities captured in their thin grids, also favouring bacterial persistence. Whilst this has long been the case in UK hospitals, in France aerators can now no longer be fitted on tap or mixer outlets.
Taps and mixers suitable for terminal filters
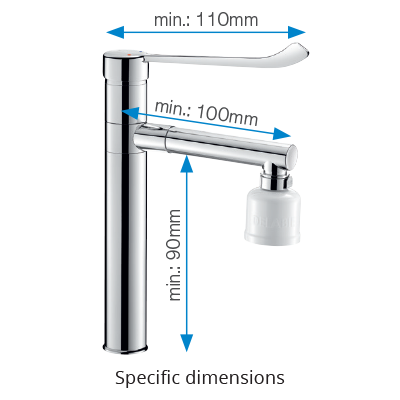
It is not enough to limit bacterial proliferation in systems, retro-contamination of bacteria present on spouts needs to be prevented. Terminal filters are commonly fitted on taps or mixers in order to provide water free of microorganisms. To facilitate the fitting of these filters without compromising user comfort, minimum dimensions have been specified: 90mm drop height (distance between the tap or mixer base and the spout) and 100mm spout length for deck-mounted controls, with at least 100mm drop height and 175mm spout length for wall-mounted versions.
Additionally, a long lever of at least 110mm is required, in order to ease grip and operation without the use of hands (generally elbows).

DELABIE at the forefront
For the last 25 years, DELABIE has been fighting for the majority of the measures contained in this French regulation to be adopted. It is, therefore, not surprising that a large part of Delabie’s healthcare range is already compliant.
With years of experience serving the UK market providing the option of copper tails, a standard option in the UK, in France was able to be done from one day to the next.
Mixers and taps from the healthcare range have flow straighteners without grids that do not inspire air. Designed in Hostaform, a material that limits the buildup of scale, their maintenance is also limited.
Some of DELABIE’s taps and mixers are fitted with a hygienic BIOSAFE outlet. Their smooth interior prevents impurities and scale deposits from being able to attach themselves therefore reducing the appearance and spread of bacteria and waterborne germs.
Most importantly, DELABIE has developed a unique patent enabling us to produce thermostatic mixers with hot and cold water closing directly on the inlets, making non-return valves redundant. As such, the risk of cross flow between hot and cold water is completely eliminated.
This is how the new H9769 dual control H9769 dual control thermostatic shower mixer is able to meet the requirements of the new regulations, as is the complete sequential thermostatic mixer range.
A number of electronic controls with the mixing chamber upstream of the closing mechanism are also equipped with this technology and, therefore, compliant with the new French regulation.
In the UK it is not currently a requirement that closing mechanisms be located upstream of the mixing chamber in mixers but the benefits are clear to see. This technology eliminates a major risk of bacterial proliferation and, therefore, is a welcome development.